Alkyne acidity and carbon carbon bond formation
Abstract
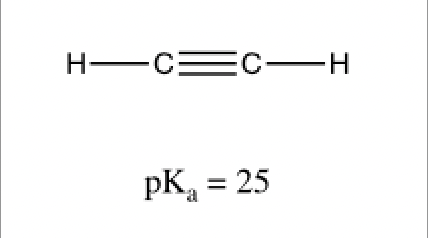
This activity introduces student to the effects of hybridization of carbon atoms on the acidity (pKa value) of the C-H bond. The activity then discusses how the acetylide ion can be used as an effective nucleophile in SN2 reactions with methyl and primary substrates. Upon completion of this activity students should understand relative acidity of C-H bonds on carbons with different hybridizations and demonstrate how to form and use acetylide ions as nucleophiles in SN2 reactions.
Level: Undergraduate
Setting: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: Organic chemistry
Keywords: alkynes, SN2, substitution, acid/base, acetylide ions, nucleophile, pKa
Downloads
Published
How to Cite
Issue
Section
License
Copyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

